This website uses cookies so that we can provide you with the best user experience possible. Cookie information is stored in your browser and performs functions such as recognising you when you return to our website and helping our team to understand which sections of the website you find most interesting and useful.
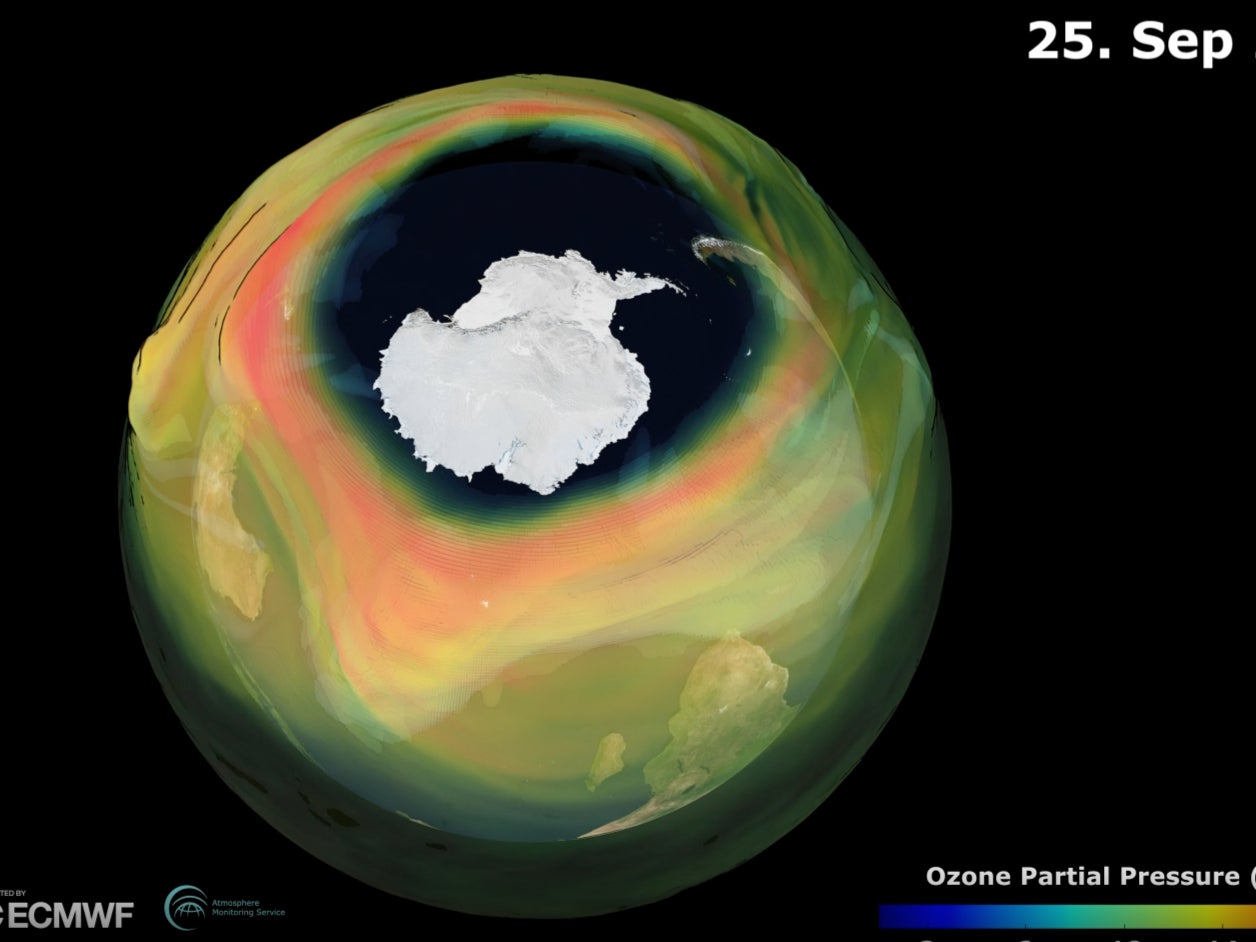
The ozone hole over the Antarctic has reached its maximum annual size and is one of the largest and deepest in recent years, scientists monitoring the protective shield have warned.
The ozone layer, in the upper part of the Earth’s atmosphere, absorbs most of the incoming ultraviolet radiation from the sun that would otherwise be damaging to life on our planet.
Concentrations of ozone, also known as trioxygen, fell to “near zero values” over Antarctica at around 20 - 25km altitude, according to scientists at the Copernicus Atmosphere Monitoring Service (Cams).
The large hole comes after an “unusually small and short-lived” ozone hole in 2019, due to particular atmospheric conditions, Cams said, and highlighted the need to enforce bans on human-made chemicals such as chlorofluorocarbons (CFCs), which are known to contribute to ozone depletion.
The scientists said this year’s large hole in the layer was being driven by a “strong, stable and cold polar vortex” - a persistent region of low pressure, usually found over the edge of the enormous Ross ice shelf, where temperatures can plunge to almost -80C.
“There is much variability in how far ozone hole events develop each year. The 2020 ozone hole resembles the one from 2018, which also was a quite large hole, and is definitely in the upper part of the pack of the last fifteen years or so”, said Vincent-Henri Peuch, director of Cams.
“With the sunlight returning to the South Pole in the last weeks, we saw continued ozone depletion over the area.
“After the unusually small and short-lived ozone hole in 2019, which was driven by special meteorological conditions, we are registering a rather large one again this year, which confirms that we need to continue enforcing the Montreal Protocol banning emissions of ozone depleting chemicals.”
The scientists explained that the hole in the ozone layer is formed as chlorine and bromine-containing substances accumulate within the freezing polar vortex, where they remain inactive in darkness.
But when the sun rises over the pole following the winter darkness, the energy it releases causes the temporarily inert bromine and chlorine atoms to become chemically active, and they rapidly destroy ozone molecules, depleting the layer.
At ground level, ozone - a pungent gas which smells similar to chlorine - is considered a pollutant as it is a powerful oxidant, which causes damage to mucous and respiratory tissues in animals, and can also impact plants.
However, its presence in the stratosphere is beneficial due to its ability to absorb medium-frequency ultraviolet light. The ozone layer absorbs around 97-99 per cent of all UV light, preventing skin cancer and other conditions.



 Africana55 Radio
Africana55 Radio 
